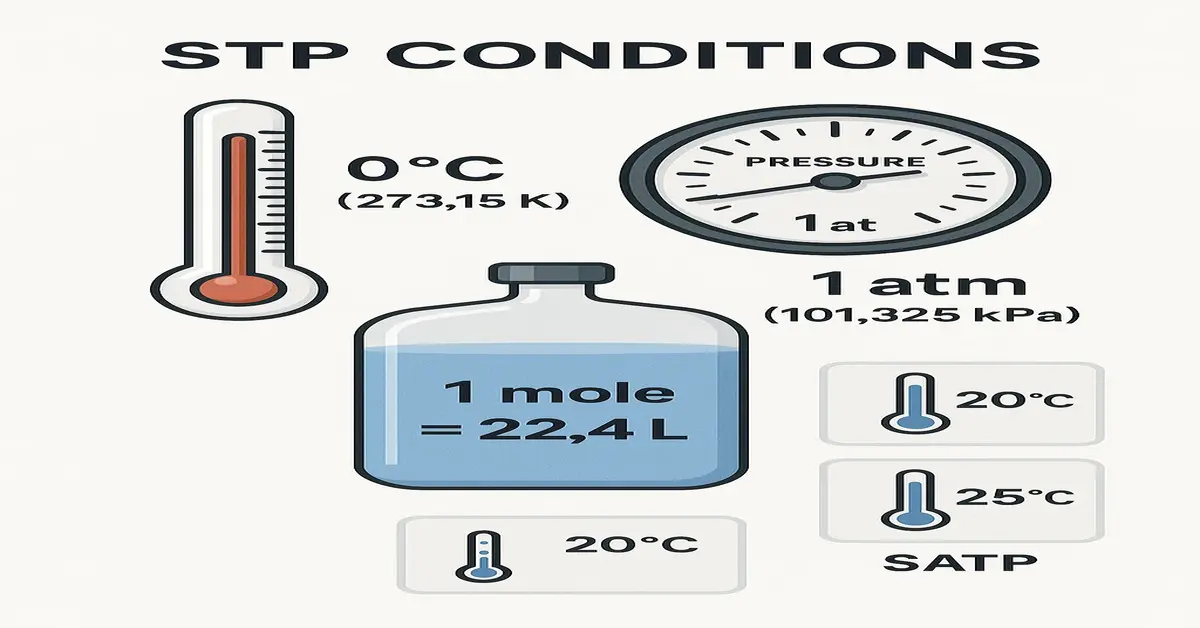
In the world of science, especially in chemistry and physics, you’ll often come across the term STP conditions. It sounds technical, but it’s quite simple once you break it down. STP stands for Standard Temperature and Pressure. Scientists use this as a common reference point so that everyone gets consistent results when measuring gases and performing calculations.
What Does STP Mean?
STP is short for Standard Temperature and Pressure. These are fixed values that scientists agree to use when doing experiments or calculations involving gases. By keeping temperature and pressure the same, scientists can compare results more fairly and predict how gases will behave. The values are:
- Standard Temperature: 0°C or 273.15 Kelvin
- Standard Pressure: 1 atmosphere (atm) or 101.325 kilopascals (kPa)
So whenever you hear someone talk about gas at STP, they mean it’s measured at 0 degrees Celsius and 1 atm pressure. This helps keep things consistent, whether the experiment is done in Pakistan, the USA, or Japan.
Why Are STP Conditions Important?
Imagine trying to compare two gas samples measured under different temperatures and pressures. You’d get confusing results, and it would be hard to say which gas behaves in which way. This is where STP helps. Scientists decided long ago to use fixed temperature and pressure values as a standard baseline for comparison. It allows: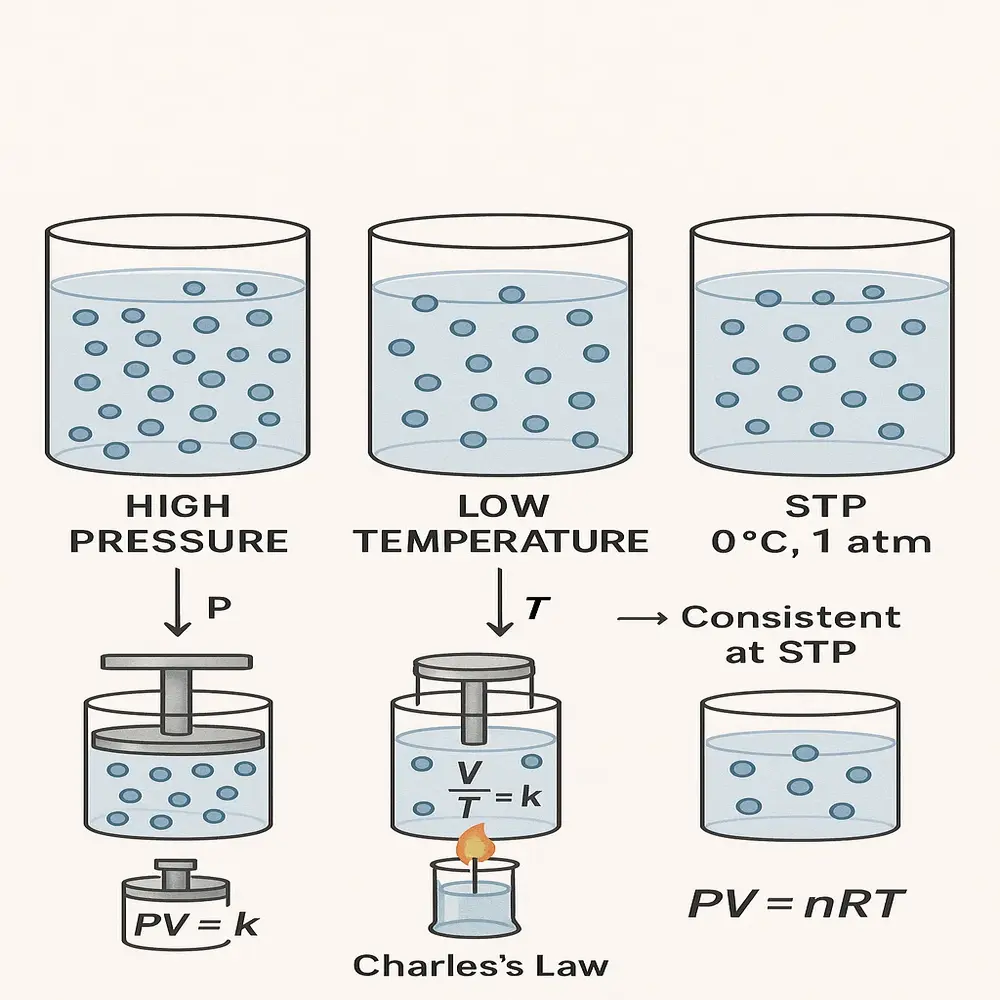
- Easier comparison of gas properties
- Precise application of gas laws such as Boyle’s Law, Charles’s Law, and the Ideal Gas Law becomes easier under STP conditions.
- Simpler classroom teaching and textbook problems
- Consistency in chemical equations and molar volume calculations
STP in Chemistry: Real-Life Applications
In chemistry, gases are a major topic. Whether it’s hydrogen in a fuel cell or oxygen during a combustion reaction, gases behave differently depending on temperature and pressure. At STP conditions, the behavior becomes predictable. This is why textbooks and lab manuals almost always give data “at STP.”
Here’s where STP becomes useful in chemistry:
- Calculating molar volume: The molar volume of an ideal gas at STP is standardized; one mole occupies approximately 22.4 liters under these conditions.
- Balancing chemical equations: Using STP makes mole-to-volume conversions easy
- Determining gas density: At standard temperature and pressure (STP), a gas’s density is closely related to its molar mass, which simplifies calculation processes significantly.
- Gas collection over water: STP allows easy comparison with vapor pressure data
- Industrial gas reactions: In industrial applications like the Haber process for ammonia synthesis, initial calculations are performed assuming STP conditions before scaling to actual operating environments.
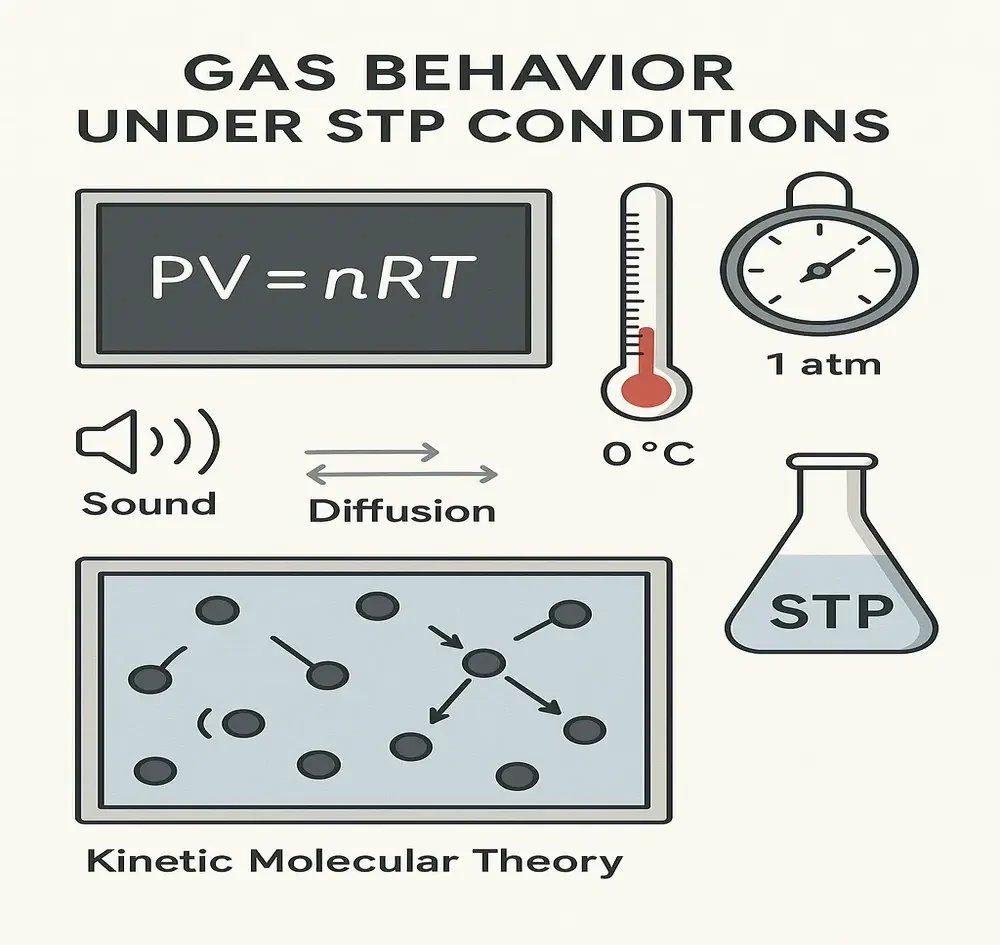
STP in Physics: Making Gas Laws Work
Physics often deals with how particles move and behave. Gases are made of billions of tiny molecules zooming around, and their speed changes with temperature and pressure. At STP, this behavior becomes more predictable. It’s easier to study motion, energy, and pressure changes when you fix the temperature and pressure.
Here’s how physics uses STP:
- Kinetic molecular theory: The speed of gas particles is measured at STP for base reference
- Ideal gas law: PV = nRT calculations often use STP to simplify the values
- Thermodynamic experiments: Starting with STP makes it easier to spot energy changes
- Sound speed in air: The speed of sound in gases is typically measured at STP to ensure uniformity across experimental conditions.
- Gas velocity and diffusion: These experiments require standard conditions for fair comparison
The Ideal Gas Law and STP
One of the most important equations in chemistry and physics is the ideal gas law:
PV=nRT
Where:
- P is pressure
- V is volume
- n is the number of moles
- R is the gas constant
- T is temperature (in Kelvin)
At STP:
- P = 1 atm
- T = 273.15 K
- R = 0.0821 L·atm/mol·K
This simplification allows for quick and accurate gas volume calculations, especially when conditions are set to STP. For example, 1 mole of gas at STP gives:
V=nRT/P=(1)(0.0821)(273.15)≈22.4L
This is why chemistry students memorize “1 mole = 22.4 L at STP.”
STP vs NTP: Are They the Same?
Not exactly. Many students confuse STP with NTP, which stands for Normal Temperature and Pressure. The main distinction lies in the specific temperature values defined for each standard condition.
- STP: 0°C (273.15 K) and 1 atm
- NTP: 20°C (293.15 K) and 1 atm
Industries often use NTP because it reflects typical room temperature conditions. But most academic textbooks stick with STP for consistency in theory and examples.
Common Mistakes Students Make With STP
Here are some things students often get wrong:
- One common mistake is using Celsius in gas law equations. Always use temperature in Kelvin when applying gas laws, as it ensures accurate and reliable results.
- Assuming molar volume is always 22.4 L (It’s only true at STP)
- Mixing up STP and NTP values
- Forgetting to adjust pressure units (Must be in atm, not mmHg or Pa unless converted)
Being careful with these details helps avoid errors and makes your work more accurate.
How to Remember STP Easily?
Here’s a quick tip:
- S for Standard
- T for 0 degrees Celsius or 273 K
- P for 1 atm pressure
Or use this fun sentence: “Standard Things Pressure us all the same.”
Fun Real-Life Examples of STP
Let’s make things even simpler with a few relatable examples:
- When you inflate a balloon in a science lab, the volume it reaches is often based on STP calculations.
- Meteorological centers standardize atmospheric readings using STP, enabling accurate data comparison across geographic locations.
- Soda manufacturers fill carbon dioxide into bottles using STP as a reference to maintain the right level of fizz.
- Scuba divers compare tank pressures with STP to help prevent decompression sickness.
Real Gases vs Ideal Gases at STP
Theoretically, one mole of an ideal gas occupies approximately 22.4 liters when measured under standard temperature and pressure conditions. But in real life:
- Real gases deviate slightly due to intermolecular forces
- When subjected to high pressure or low temperature, gases tend to deviate from ideal behavior due to intermolecular interactions.
- But at STP, the difference is usually negligible for basic calculations
Conclusion
STP conditions help scientists, teachers, students, and industries work with gases in a controlled and comparable way. By fixing temperature and pressure at known values, everyone has a shared starting point for experiments, calculations, and understanding how gases behave. From classroom equations to real-world gas production, STP makes the science of gases consistent, simple, and universal.