In the world of clinical research, data is everything. How it’s collected, managed, and analyzed can make or break the success of a clinical trial. That’s why investing in the right clinical data management system (CDMS) has become a priority for research teams in 2025.
A modern CDMS does more than just store data. It improves trial performance, ensures clinical trial compliance, and reduces risk by integrating intelligent features like AI, cloud access, and real-time monitoring.
What a Clinical Data Management System Does?
A Clinical Data Management System (CDMS) is designed to simplify and optimize how clinical trial data is collected and managed. It helps minimize manual work by reducing the time spent on entering and verifying data, ensuring that information is complete, consistent, and ready for accurate analysis.
Modern CDMS platforms now include advanced technologies like artificial intelligence and machine learning, which can automatically detect discrepancies or unusual patterns in the data.
Modern CDMS platforms support a wide range of tasks including:
- Designing Case Report Forms (CRFs)
- Automating data validation
- Managing audit trails
- Generating real-time reports
- Integrating with other clinical systems
Why the Right Tools Matter in 2025?
The tools built into a CDMS can make or break the success of a clinical trial. With the rise of decentralized and hybrid trials, real-time data access and automation have become essential.
Choosing the right CDMS tools means:
- Faster patient data processing
- Fewer errors during validation
- Improved compliance with global regulations
- Better communication between teams
- Shorter trial cycles and quicker approvals
1. Cloud-Based CDMS
With the shift toward remote and hybrid clinical trials, cloud technology has become the backbone of modern data systems. A cloud-based CDMS allows research teams to log in from any location and manage data in real time.
This eliminates delays caused by limited on-site access and enables secure clinical data storage with automated backups and system updates.
Key benefits include:
- 24/7 global accessibility
- Seamless collaboration between sites
- Built-in disaster recovery
- Scalability for trials of any size
- Reduced infrastructure costs
Cloud access also supports decentralized trial models where participants contribute data from home via mobile apps or digital health platforms.
2. AI-Powered Trial Data Validation
In 2025, AI in clinical trials is no longer optional but it’s essential. Validating data manually takes time and increases the risk of human error. Modern CDMS platforms now include AI-powered features that automate query generation, data checks, and error flagging.
Machine learning algorithms can detect unusual patterns, missing fields or inconsistencies instantly, allowing teams to correct problems early and maintain real-time clinical data quality.
AI capabilities include:
- Smart data validation and correction
- Predictive data modeling
- Auto query resolution
- Data trend forecasting
- Workflow automation
This level of intelligence not only speeds up clinical trials but also improves decision-making throughout the study lifecycle.
3. GCP & FDA-Compliant Audit Trails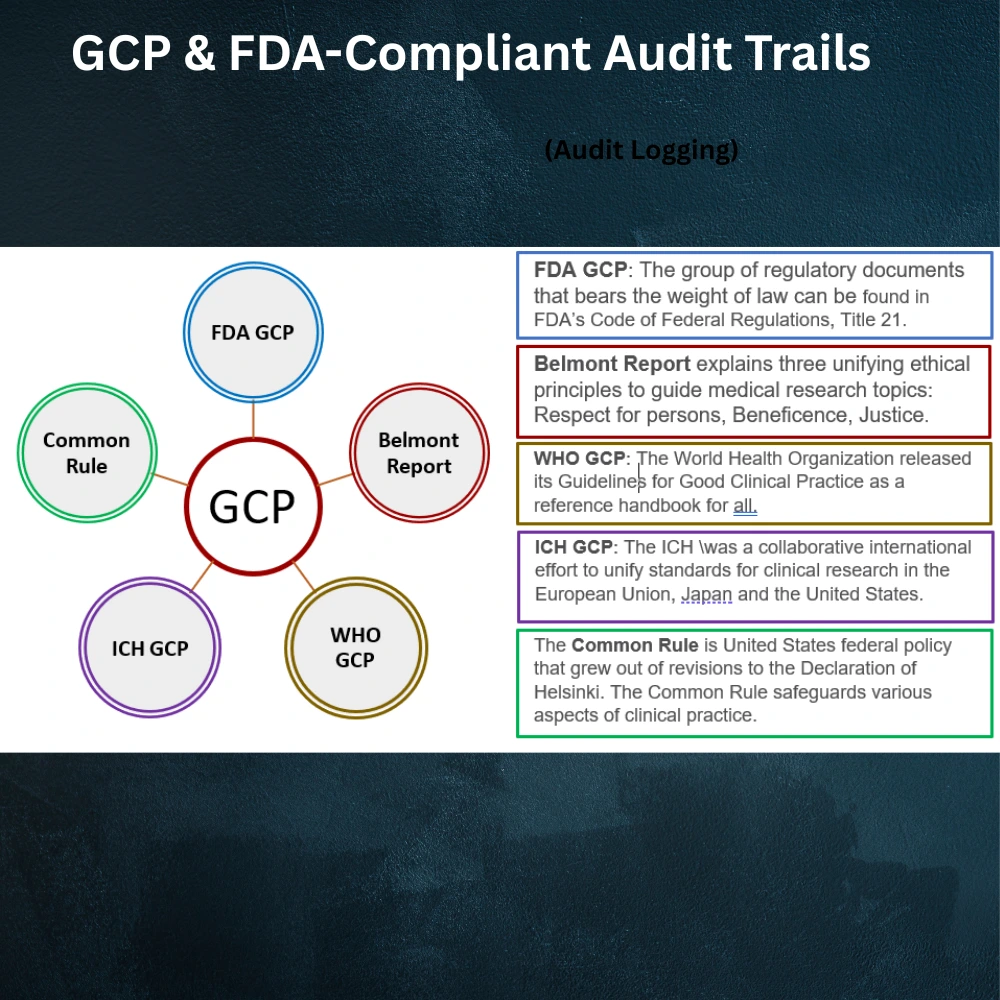
When it comes to regulatory compliance, a CDMS must align with global standards like Good Clinical Practice (GCP) and FDA 21 CFR Part 11. These guidelines require full traceability and transparent data handling processes.
That’s why modern CDMS platforms are designed with automated audit trails that log every action who did what, when, and why.
Compliance-ready features include:
- Secure electronic signatures
- Role based user access control
- Time stamped change logs
- Protocol deviation alerts
- Locking and freeze functions for finalized data
These tools protect against regulatory penalties and support seamless audit preparation, giving sponsors and regulators confidence in data integrity.
4. Seamless Integration with EHR, ePRO, and Lab Systems
Gone are the days of managing trial data in silos. A next-gen CDMS must connect effortlessly with Electronic Health Records (EHR), lab systems, electronic patient reported outcomes (ePRO) and wearable devices.
CDMS integration with EHR and other systems reduces duplication, ensures consistency and supports a more holistic patient data view.
Common integration benefits:
- Unified patient data across platforms
- Faster data import/export
- Live sync with lab and diagnostic tools
- Automation of data entry tasks
- Accurate reporting with fewer errors
This creates a smoother workflow and strengthens the clinical data pipeline from source to analysis.
5. Intuitive and Customizable User Dashboards
The best clinical trial software doesn’t just work well also it’s easy to use. One of the most requested features in 2025 is a user-friendly CDMS dashboard that’s customizable based on individual roles and workflows.
Whether you’re a data manager, CRA or sponsor, having quick access to relevant KPIs, queries and site statuses improves focus and productivity.
Top usability features include:
- Drag-and-drop form builders
- Visual analytics and charts
- Personalized home screens
- Mobile responsiveness
- Role specific alerts and views
By simplifying navigation and eliminating clutter, these clinical research software tools make it easier to manage complex trials with confidence.
6. Role-Based Access and Security
Data privacy and protection are critical in clinical research. Modern CDMS tools include role-based access control that restricts what each user can view or change based on their job.
Security features may include:
- Two-factor authentication
- Encrypted data transmission
- Custom user roles and permissions
- Activity logging for every user
These safeguards ensure compliance with data protection laws and reduce the risk of unauthorized access.
7. Built-In EDC Capabilities
Many CDMS platforms now include Electronic Data Capture (EDC) tools as part of the system. This makes it easier to collect patient data electronically using web forms, mobile devices, or tablets.
EDC features often include:
- Form version control
- Edit checks during data entry
- Visit scheduling and tracking
- Offline data entry options
Combining EDC and CDMS tools into a single system reduces costs and simplifies the trial process.
8. Support for Decentralized Trials
With the rise of virtual trials, CDMS tools must now support decentralized trial models. This means handling data from mobile apps, wearable devices, and home-based monitoring tools.
Key capabilities include:
- Mobile-compatible data entry interfaces
- Patient portals for remote participation
- Integration with digital health platforms
- Real-time notifications and alerts
This flexibility helps boost patient engagement and allows trials to continue even when in-person visits are limited.
9. Custom Workflow Automation
Automation is a game-changer in clinical trials. CDMS tools can be configured to automate routine tasks such as:
- Generating queries
- Sending email reminders
- Locking completed forms
- Exporting datasets on a schedule
Automated workflows reduce human error, save time, and let staff focus on more important tasks.
10. Centralized Query Management
Handling queries across sites can be overwhelming. Modern CDMS platforms include centralized query management tools that make it easy to track, respond to, and close queries.
These tools help:
- Reduce query response times
- Improve communication with sites
- Ensure all discrepancies are addressed before database lock
This feature is especially useful in large, multi-site studies where communication needs to stay organized.
CDMS Features vs. Traditional Methods
As clinical research evolves, the limitations of traditional data management methods like spreadsheets, manual logs and disconnected systems have become more apparent. In contrast, modern Clinical Data Management Systems (CDMS) offer smarter, faster and more integrated solutions. The table below highlights key differences between outdated approaches and the advanced features found in today’s CDMS platforms.
Why CDMS Tools Matter in Clinical Trials?
| CDMS Tool | Description | Key Benefit |
| Cloud-Based Access | Enables remote, real-time data management | Flexibility and instant collaboration |
| AI-Powered Data Validation | Detects inconsistencies using artificial intelligence | Faster data cleaning and fewer errors |
| Regulatory Audit Trails | Logs every user action with time stamps | GCP and FDA compliance readiness |
| Custom Dashboards & Reports | Role-based, real-time visual summaries | Faster insights and better monitoring |
| Integration with EHR, LIMS, ePRO | Connects with other systems for seamless data flow | Reduced duplication and more complete data |
A Clinical Data Management System (CDMS) plays a critical role in the success of modern clinical trials. But it’s not just about having a system, it’s about having the right features. These features help research teams manage complex trial data more efficiently, meet regulatory standards and ensure that the data collected is clean, accurate and ready for analysis.
The better the features, the smoother the trial process from data entry to database lock and final submission.
Here’s why CDMS features are so important:
- Data Accuracy: Real-time validation and query management reduce human error and ensure cleaner, more reliable trial data.
- Regulatory Compliance: Built-in audit trails and user access controls support alignment with FDA, EMA and ICH-GCP guidelines.
- Faster Decision-Making: Live dashboards and reporting tools allow for quicker interim analyses and Data Safety Monitoring Board (DSMB) reviews.
- Workflow Efficiency: Automated processes like discrepancy management and auto-query generation reduce manual workload and delays.
- Integration Capabilities: Seamless connection with EHR, ePRO, labs and wearables consolidates all patient data in one place.
- Secure Data Storage: Cloud-based CDMS ensures secure backups, encrypted data and disaster recovery readiness.
- Support for Remote Trials: Features like mobile access and role-based dashboards enable decentralized or hybrid trial models.
When clinical teams have access to a CDMS with modern, user-friendly, and powerful features, they gain better control over trial timelines, data integrity and overall study outcomes. These features are not just helpful also they’re essential in meeting the demands of today’s research landscape.
Final Thoughts
As clinical trials become more advanced, the tools we use to manage them must evolve too. A modern Clinical Data Management System is more than a storage solution, It’s a dynamic platform that supports every phase of the research process.
From cloud access and AI validation to system integration and real-time dashboards, the essential CDMS tools for clinical trials in 2025 are built to streamline workflows, improve data quality and ensure compliance with ever-changing regulations.
Investing in the right CDMS features doesn’t just improve efficiency. it protects the integrity of your study and helps bring life-changing therapies to patients faster.



